Ofev Benefits IPF Patients Regardless of Initial Prognosis, Trials’ Data Show
Ofev (nintedanib) can prevent disease progression in people with idiopathic pulmonary fibrosis (IPF) regardless of their prognosis at treatment start as staged by the GAP system, a new analysis of results from the Phase 3 INPULSIS trials show.
The authors of this mathematical study analyzed already published data that was pooled together from the two INPULSIS trials. No new clinical trials were conducted. The results were published in the journal ERS Open Researchin the report, “Effects of nintedanib in patients with idiopathic pulmonary fibrosis by GAP stage.”
INPULSIS-1 (NCT01335464) and INPULSIS-2 (NCT01335477) were two pivotal Phase 3 clinical studies designed to confirm the efficacy and safety of Ofev in preventing or slowing IPF progression. These studies compared outcomes of IPF patients who took 150 mg of Ofev twice daily with those who received a placebo, for a treatment period of 52 weeks.
In this new re-analysis of the trials’ data, patients were grouped according to their GAP stage at the study’s start. The groups were GAP stage I (better prognosis) or GAP stage II/III (worse prognosis).
GAP is a risk prediction index that estimates prognosis of a person with IPF based on the patient’s gender (G), age (A), and two lung physiology (P) variables — forced vital capacity (FVC) and diffusing capacity of the lung for carbon monoxide. The GAP index is designed to predict the average risk of a patient’s mortality in the next 1, 2, and 3 years. The creators of the GAP index suggested that it could be used to select a certain risk group of patients for enrollment in clinical trials.
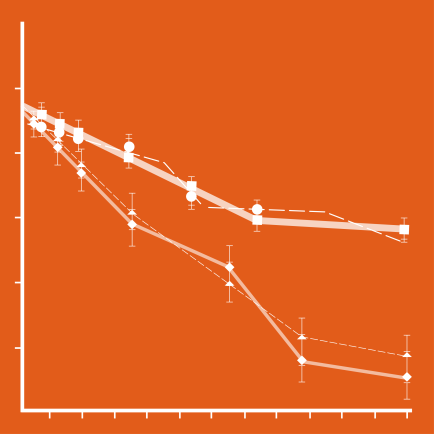
Here, researchers reviewed the clinical records of 1,060 patients with IPF who participated in the INPULSIS trials. Before starting treatment with Ofev or placebo, 500 patients were at GAP stage I, 489 were at GAP stage II, and 71 were at GAP stage III.
Results showed that the rate of disease progression, as measured by the annual decline in FVC, was similar between patients at GAP stage I and those at GAP stage II/III who were treated with Ofev.
The efficacy of Ofev in delaying FVC decline in these patient groups also was consistent with the treatment effect in the overall population of patients, as shown by previously published results of INPULSIS trials. This confirms the efficacy of the treatment and its beneficial effect on slowing disease progression compared to placebo.
“This post hocsubgroup analysis of pooled data from the INPULSIS trials suggested that [Ofev] had a similar benefit on the rate of FVC decline across patient subgroups stratified by baseline GAP stage I versus II/III,” the researchers stated.
Ofev’s positive effect was also found to be consistent across different patient GAP stages when using other measures of disease progression. In the GAP I group, an absolute FVC decline of at least 10% or death was experienced by 39.8% of patients who received placebo and 24.7% of GAP I patients treated with Ofev. In the GAP II/III group, an absolute FVC decline of at least 10% or death was experienced by 42.9% of placebo-treated patients and 29.3% of Ofev-treated patients.
There were fewer deaths reported in this study than were predicted by GAP staging at the beginning of the study. There were 79.9 deaths predicted in the Ofev group and 55.2 in the placebo group. However, during the trial, there were actually 35 reported deaths in the Ofev arm (43.8% of expected) and 33 reported deaths in the placebo arm (59.8% of expected).
This suggests that patients in the INPLUSIS trials had lower mortality rates overall than the general population of patients with IPF, researchers said. In addition, treatment with Ofev was “associated with a relative reduction in the risk of mortality of 26.7% compared with placebo,” they said.
These findings extend the results of previous studies demonstrating the benefit of Ofev across many subgroups of patients with IPF. Also, the team believes that they show that GAP staging may not be a useful way to select patients for clinical trials testing anti-fibrotic therapies.
“GAP stage is unlikely to be a helpful tool for cohort enrichment in similarly designed clinical trials of pharmacotherapies for IPF,” they said.
The researchers noted that some GAP stage-specific differences were seen in quality of life, time to first acute exacerbation, and rate of Ofev discontinuation. Although these differences were numerically greater with increasing GAP stage, they were not statistically significant when comparing the GAP I group against the GAP II/III group. Additional studies are needed to understand if these differences are meaningful or clinically relevant.
Adapted from pulmonaryfibrosisnews.com